Clinical Trial Management Software as a Service
In 2020, 362,552clinical studies were registered globally, and the number is steadily growing. During the COVID-19 pandemic, clinical trials became more important than ever. The market for clinical trial software is expected to grow at a CAGR of 14.7% and reach USD $1,590 million by 2025.
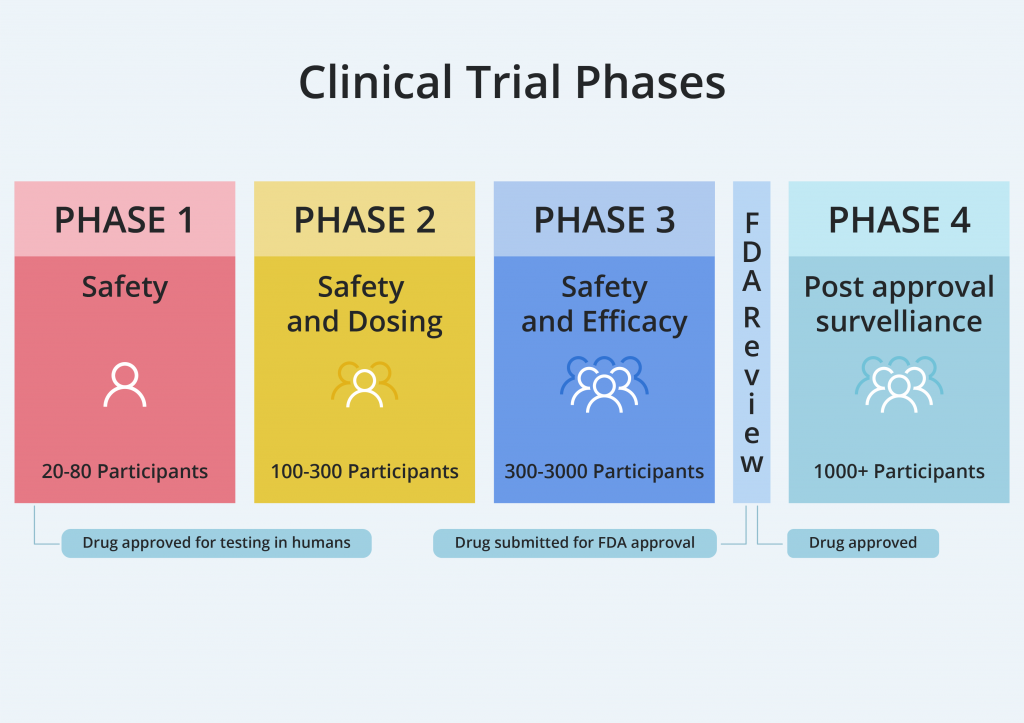
A clinical trial is a complex process that usually consists of 3 stages varying in duration, the number of participants, and goals.
The entire process requires lots of data management, paperwork, and communication, all of which distract from the clinical research itself. What does research and development do to automate these routine processes?
A CTMS (clinical trials management system) may provide a solution, and in this article, we discuss the main benefits, features, and stages of such software development.
The Purposes and Configurations of Clinical Trial Management Software
What is CTMS used for? Biotech and pharmaceutical companies utilize CTMS for managing clinical trials. It helps researchers and Clinical Research Organizations (CROs) organize all the steps of the clinical trial process from creating a list of volunteers to analyzing the achieved results.
Other facilities where clinical research is conducted, such as research hospitals, physician practices, academic medical centers, or cancer centers, also widely utilize CTMS.
Clinical trial management holds the possibility for automated patient recruitment. Usually, the companies look for people through traditional channels: TV, print media, and radio, so a massive amount of candidates may apply and researchers need to filter out the unfitting ones. With clinical research software, a CRO can easily detect applicants who meet their requirements and send them detailed information about current trials.
CTMS is also often used for facilitating siteless trials. Almost 70% of potential research participants live more than 2 hours away from a trial site. Nurses arrange a meeting with participants at home. If bulky equipment is needed (X-Rays, MRIs, etc.), the clinical trial software helps plan the visits and allows researchers to take the material and conduct the remaining steps without physical contact. Siteless trials save the participants’ time, reduce travel-related costs, and allow working with homebound patients.
With the help of CTMS, clinical trials can be monitored in real-time. It helps to analyze progress, detect potential problems, and set a financial goal.
When the number of patients and tests are small, organizations can make do with basic in-house systems handling their data. As data volumes and complexity grow, the need for more comprehensive software arises. The sponsors of clinical trials may provide such CTMS to the sites that participate in their trials, or the sites may use a software of their choice to support their day-to-day operations. Some systems require dedicated servers, but cloud-based CTMS are more popular. The latter are marketed as software as a service (SaaS).
Let’s dive deeper into the main benefits of building such a solution.
The Benefits of Creating a Clinical Trials Management Platform
All parties at clinical trials – CROs, researchers, participants, and sponsors – may benefit from utilizing a CTMS. Here are some of its main advantages:
Efficient Data Management
Efficient data management is of crucial importance for CROs, so they are willing to pay for quality software that would help to ensure it.
When conducting a clinical trial in a traditional way, researchers focus on the trial as a whole. Being fully immersed in the process, they may fail to properly structure the wealth of information. For example, they may forget to name a material, causing inaccuracies in subsequent data. CTMS reduces the risk of missing any of the details. Every piece of information regarding a particular trial is located within one platform and can be quickly updated in real-time.
Clinical research software collects and structures data in one place allowing to find any document within a minute. With CTMS in place, a CRO will not mix up the results of different trials as it records every case separately. By providing up-to-date details to study information, CTMS improves the transparency for researchers.
Protocols Compliance
The importance of data security and regulatory compliance in the healthcare sector can’t be overestimated. The financial penalties for violating legal requirements can be significant. For example, if a research organization doesn’t meet the one-year deadline for reporting trial results to ClinicalTrials.gov, penalties reach $12,103 per day.
Clinical trial management software helps to ensure compliance with all the legal protocols and reminds researchers about important deadlines. The software also automatically updates the trial team, partners, and federal organization about the latest outcomes. CTMS promotes collaboration by bonding internal and external parties together and guarantees that the trial goes legally.
Research organizations will be likelier to invest in software that helps avoid massive fines than run the risk of paying them and losing their reputation.
Strong Security
It is essential to keep clinical trial information protected from third parties. CTMS provides different access levels to researchers and enforces multi-factor authentication, strong passwords, and other security measures.
SaaS management software for clinical trials goes further and offers secure messaging, secure cloud storage, virus protection, data encryption, and server monitoring – and their clients are prepared to pay for these advantages.
Transparent Financial Management
US FDA reports that the median cost of a clinical trial is $19 million; in every case, the amount mainly depends on the number of studies. They are usually funded by sponsors who request reports about the budget allocation. Using clinical trial administration software, research organizations can track every single receipt and pursue a transparent financial policy.
Furthermore, according to the Sunshine Act, the manufacturers of drugs, medical devices, and supplies should report on the financial side of relationships with physicians to Centers for Medicare and Medicaid Services. CTMS makes it easier to deliver the information in a legal way.
Minimized Risk of Mistakes
Another benefit of clinical research software is accuracy in storing the data. The statistics say that only 13.3% of analyzed trials were conducted with no error, while others made at least one mistake. Even one mistake may jeopardize the whole clinical trial. The functions of CTMS are fully reliable, so researchers will have more time to concentrate on the trial.
Again, the cost of implementing a CTMS cannot be compared with a wasted research budget, fines, and the loss of future profits that errors may cause.
Preventing Multiple Trial Enrolment
A patient’s enrollment in multiple trials may negatively affect the results of the research. Clinical research administration software utilizes biometric technologies such as fingerprints or facial recognition to detect patients who are currently participating in another trial and prevent their enrolment. It excludes the possibility of money and time waste caused by professional clinical trial participants.
Saved resources
An average clinical trial can take up to 6-7 years. A solid STMS solution accelerates the R&D process.
Improving Personnel Management
CTMS often includes features of employee benefits software and simplifies the process of managing researchers, their payrolls, and benefits for meeting deadlines or other achievements.
Benefits administration software also streamlines the process of recruiting, managing, and paying the participants of a study.
The pharmaceutical research and development process is long and may present many challenges. CTMS developers should also know about them and offer solutions that can give a competitive edge.

Clinical Trial Challenges and Their Solutions
Specialists conducting clinical trials need to be connected with multiple systems and data resources. This is where the lack of uniformity appears. To prevent distraction on questionnaires, apps, forms, and other systems, they should choose the place where every result will be recorded.
Over the past decades, clinical trial data management has made a switch from paper documents to innovative forms, but the process of integration remains not automated. Specialists will appreciate a CTMS system that can bond all the sources together.
Adopting Clinical Data Standards by CDISC
CDISC (Clinical Data Interchange Standards Consortium) is a standards-making organization that allows data capture and exchange in clinical trials. They develop free and fully customizable standards which are suitable for every company.
One of the most common CDISC standards is the Study Data Tabulation Model (SDTM) that defines how to organize the collected data into tables. SDTM datasets structure all the information collected during the clinical trial making it ready for regulatory submission.
Information in the SDTM format can be used for creating data models and making sense of the data collected. CDISC Analytical Data Model (CDISC ADaM) helps researchers structure and detect similar partners in the massive amount of collected information.
To make a long story short, CDISC has set numerous requirements on how clinical trial data should be captured, stored, used in analysis data model, and exchanged. It is almost impossible to ensure the correspondence to all of these requirements manually, but CTMS can easily do this automatically.
Automating EHR-EDC Connectivity with the OneSource Model
Data from healthcare cases and data from clinical trials are not collected together. This is ineffective, as the combined results of both systems are useful for later analysis and use. But there is a way where Electronic Health Records (EHR) and Electronic Data Capture (EDC) can be connected.
FDA partnered with the University of California San Francisco and Stanford Centers of Excellence in Regulatory Science and Innovation to create OneSource – a mechanism that bonds EHRs and EDC.
The idea is that both EHRs and EDC data are located in one confirmed source according to all the clinical data standards. CTMS can facilitate this process enabling researchers to have access to complete information about the patients.
Modernizing Outdated CTMS Software
The first CTMS was developed 20 years ago, and a large number of current systems still rely on the practices that have been used there. Such legacy software no longer satisfies the needs of researchers, CROs, and sponsors. There is a need to either switch to a new solution or modernize current software.
Specialists recommend following the second option because changing the entire software may slow down the work of the researchers due to a learning curve and would take some time to fully customize it. CTMS developers should be able to offer a solution that is better than the existing one, is easy to use, and is flexible enough to meet various needs of the clients.
Both health tech companies and startups and institutions that want a custom clinical trial management software need to profoundly study all the needed features to implement.
Features that a Clinical Trial Management Software Should Include
The feature set of a CTMS and the features characteristics should be determined by the needs and goals of researchers that would be using it. However, the following features are essential to any clinical research software:
Researchers’ Profiles
Both participants and sponsors should be able to easily locate general information about researchers: name, address, degrees, licenses, specialties, and so on. This feature makes the relationship transparent and reliable.
Work Processes
The software should include a section where researchers can track and analyze all the workflows related to the participants’ reactions to various manipulations.
Monitoring and Protocol Management
To avoid legal and financial consequences, a CRO needs to constantly monitor the protocols’ compliance and quickly address the detected violations. A CTMS should monitor the security of the data connected with participants and trial results. It should send notifications about potential security risks so they could be quickly addressed.
Сlinical trials also imply risks to the participants’ health. By setting triggers that send alarms if a suspicious health condition is detected, researchers can detect and solve the problem in a timely manner.
Integrations with the Clinical Trial’s Website
Clinical trial software should have integration with the website so that all parties can gain easy access to the information about the clinical trial progress, current results, payments, schedule, etc.
Secure Cloud Storage
As clinical research involves collecting a huge amount of documentation, data about the studies and the participants, it is important to upload the data into cloud storage that will be accessible for all authorized parties.
Mobile-Optimized System
You may create a web application hosted in the cloud which guarantees trouble-free access from all around the world. However, a mobile version of CTMS will increase the convenience for researchers who have to travel frequently.
Payment Processing System
This functionality should facilitate tracking the tasks, forming receipts, and making payments in one place. Traditionally, these operations can be performed in different systems, but clinical research administration software can combine all these functions.
Connection to Wearables
The application of wearable technology can reinforce the research process. Integration with wearables allows CTMS users to track patients’ health and get accurate data about patient-reported outcomes. This allows a decrease in the number of in-clinic patients and achieves cost reduction and improved subject retention.
Live chats
Consulting every participant via email or telephone may be cumbersome, and chats provide an alternative. Moreover, having all correspondence in one place helps to store the data in one place and ensure its security.

Let’s move on to the steps that would help you better define your business idea and facilitate successful development of a clinical research management solution.
5 Steps to Build CTMS
We distinguish five main steps based on Alternative-spaces teams’ experience in developing software for the healthcare industry.
1. Define the Goals
Before you start building SaaS management software for clinical trials, you need to point out the goals it should satisfy. Analyze the current issues with the administration of research and patient information, workflows, resources (including financial ones), etc. Consider a system of supporting clinical decisions and multilingual format.
Remember to take into account all applicable compliance and safety requirements.
2. Create the System Workflow
SaaS platform developers need to implement multiple functions that will cover the defined goals. Look over the platform’s interface, secure cloud storage, its multilingual version, and add the portals where the researchers can overview the data. Try to focus on CDISC standards of how to present and structure the information.
3. Develop an Efficient Design
The design of CTMS should facilitate the input, structuring, and management of massive amounts of information. Establish clear navigation, group elements by hierarchy, and make the interface as intuitive as possible.
4. Build the Software
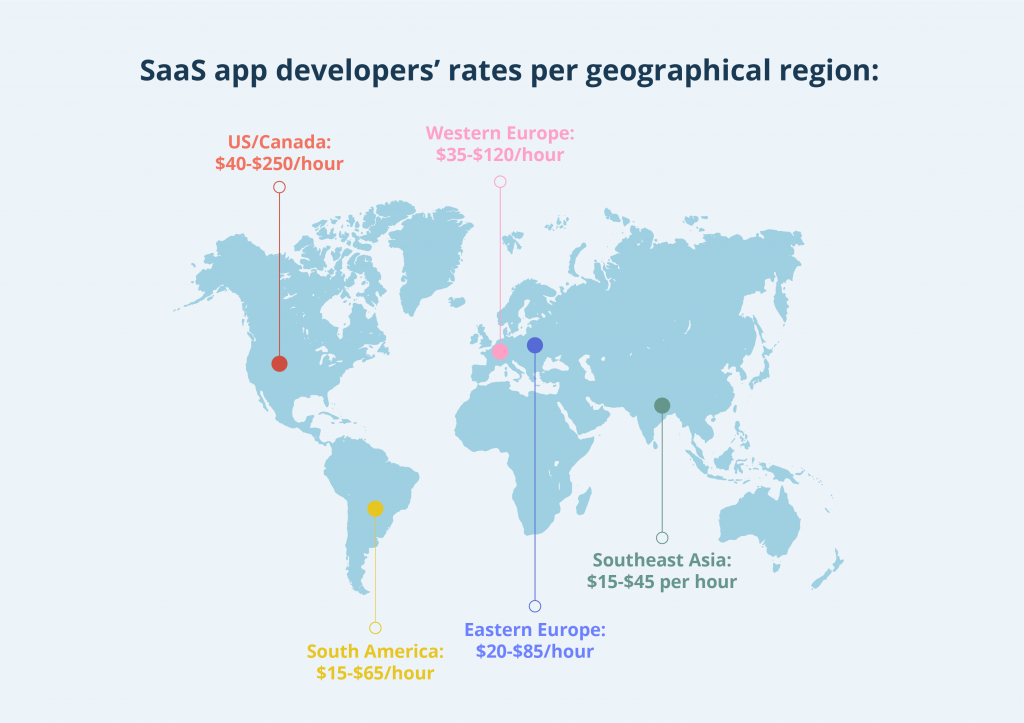
If you haven’t developed SaaS products and clinical trial software before, experienced professionals can help you to save much time and headache. If you outsource the project or engage qualified off-shore software developers, you can also save money. The professionals’ hourly rates depend on the country and company, but you need to focus on the quality first.
5. Test the SaaS Platform
Before you open your clinical trial management software to the whole world, you need to check its reliability, availability, stability, and efficiency. The data must be strongly protected and the platform should be easy to use.
Run the software and collect users’ feedback. Fix the bugs and improve the system’s elements. Be sure that nothing will endanger the research and patients’ data.
Conclusion
The implementation of clinical trial management software promises various advantages both for researchers and sponsors. With an effective CTMS in place, they can easily manage the data, ensure financial transparency, optimize the use of resources, and prevent human errors.
CTMS facilitates research reporting according to CDISC standards and proper budget calculations, and helps conduct trials in time and keep the information up to date. The software reduces the time of R&D development and addresses and solves clinical trial challenges related to lack of uniformity and integration of external and internal sides.
If you consider developing a CTMS that would successfully serve the needs of the research community and turn into a profitable business, please feel free to contact Alternative-spaces. We have years of experience developing SaaS platforms and innovative solutions for healthcare. You can rely on our expertise at all stages of your product development, from market research and idea validation to testing and maintenance.
Content created by our partner, Onix-systems.
 Home
Home